The Building Blocks
Neural Stimulation by Photovoltaic Nanoparticles
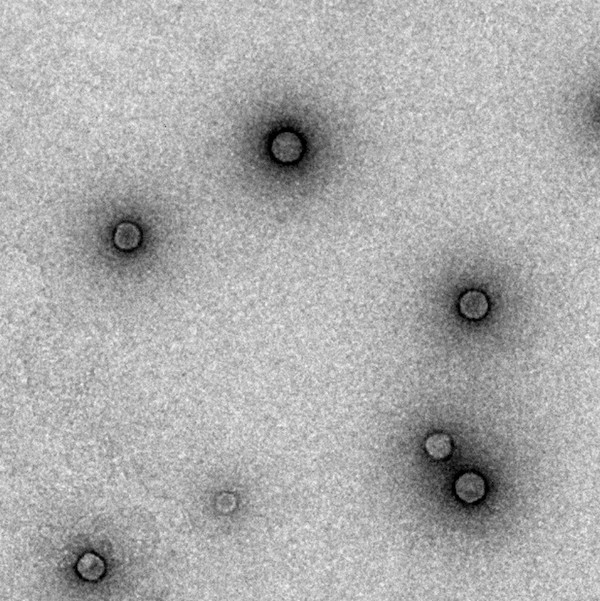
The application of optoelectronic properties, peculiar of conjugated polymers, in the neuroscience and biomedical fields represents the last and most ambitious frontier of research. Inspired by organic solar cells, IRCCS/IIT, the project leader, pioneered an interface composed of a semiconductive polymer able to excite neurons in response to visible light stimuli.
The polymer layer, subretinally implanted in rats suffering from photoreceptor degeneration, was able to rescue light sensitivity, light-driven behaviors, and visual acuity in vivo. 1-5
TEM imaging of polythiophene NPs (P3HT, 80 nm diameter)
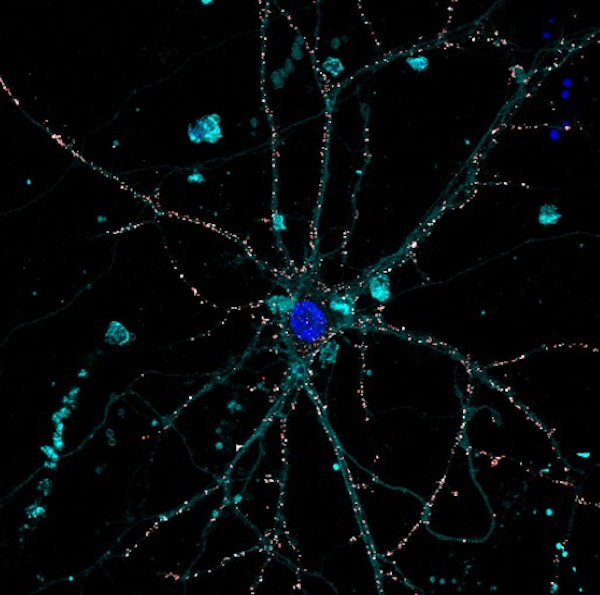
Very recently, the group we generated prototypical P3HT-NPs that microinjected in the outer retina of blind rats bearing photoreceptor degeneration, promoted light-dependent activation of the spared inner retinal neurons, recovering subcortical, cortical and behavioral visual responses.
This promoted the development of a novel therapeutic strategy for the treatment of retinitis pigmentosa and macular degeneration.
Confocal imaging of cortical neuron exposed to P3HT NPs (red)
- 1. Ghezzi, Diego, et al. “A polymer optoelectronic interface restores light sensitivity in blind rat retinas.” Nature Photonics 7.5 (2013): 400-406.
- 2. Maya-Vetencourt, José Fernando, et al. “A fully organic retinal prosthesis restores vision in a rat model of degenerative blindness.” Nature Materials 16.6 (2017): 681-689.
- 3. Benfenati, Fabio, and Guglielmo Lanzani. “New technologies for developing second generation retinal prostheses.” Lab animal 47.3 (2018): 71-75.
- 4. Maya-Vetencourt, José Fernando, et al. “Subretinally injected semiconducting polymer nanoparticles rescue vision in a rat model of retinal dystrophy.” Nature Nanotechnology 15.8 (2020): 698-708.
- 5. Maya-Vetencourt, José Fernando, et al. “Subretinally injected semiconducting polymer nanoparticles rescue vision in a rat model of retinal dystrophy.” Nature Nanotechnology 15.8 (2020): 698-708.
The M13 Phage
M13 phages are known to be ideal carriers for a variety of agents,6,7 as they are non-toxic and rapidly cleared from the peripheral circulation. Bacteriophages have been widely used to treat antibiotic-resistant bacterial infections or as filamentous phage display systems for the study of protein and peptide interactions. However, these systems also represent an innovative, harmless, and effective delivery vehicle.
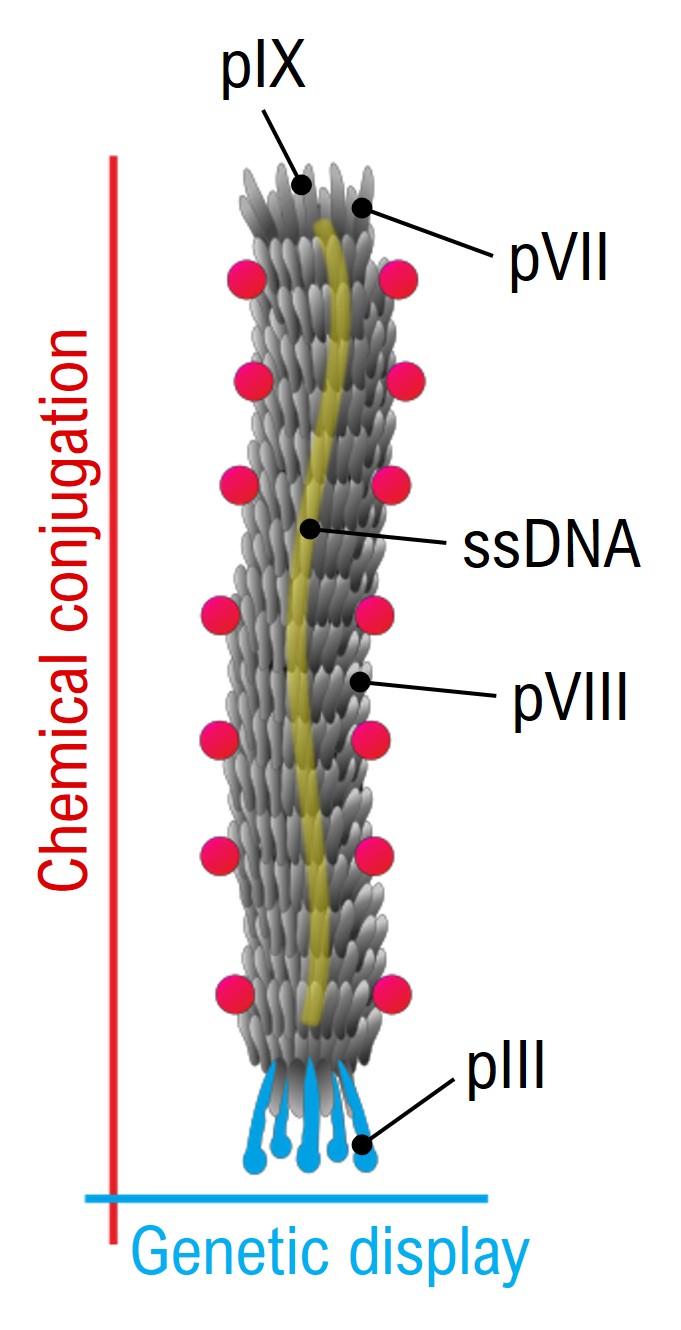
Schematic of M13 Phage structure
Genetic engineering and orthogonal modification of the two major classes of genetically encoded coat proteins, namely the minor coat pIII (present at the tip of the vector in 5 copies) and the major coat pVIII (covering the lateral surface of the vector with 2700 copies) make M13 a multifunctional and targeted carrier. These systems possess clear advantages: high avidity for a plethora of targets, cost-effective production, high loading capacity and multivalency.
Schematic of M13 Phage structure
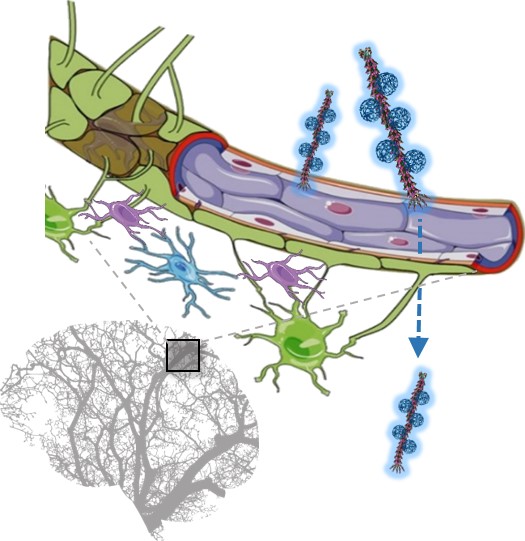
Another key advantage of the use of phages as nanocarriers consists of their efficient translocation across the BBB, which is an essential requirement for the efficacy of systemically administered treatments.
Schematic of the blood-brain barrier translocation of the hybrid M13 phage-P3HT NPs system
- 6. Ulfo, Luca, et al. “Orthogonal nanoarchitectonics of M13 phage for receptor targeted anticancer photodynamic therapy.” Nanoscale 14.3 (2022): 632-641.
- 7. Bortot, Barbara, et al. “Advanced photodynamic therapy with an engineered M13 phage targeting EGFR: Mitochondrial localization and autophagy induction in ovarian cancer cell lines.” Free Radical Biology and Medicine 179 (2022): 242-251.

